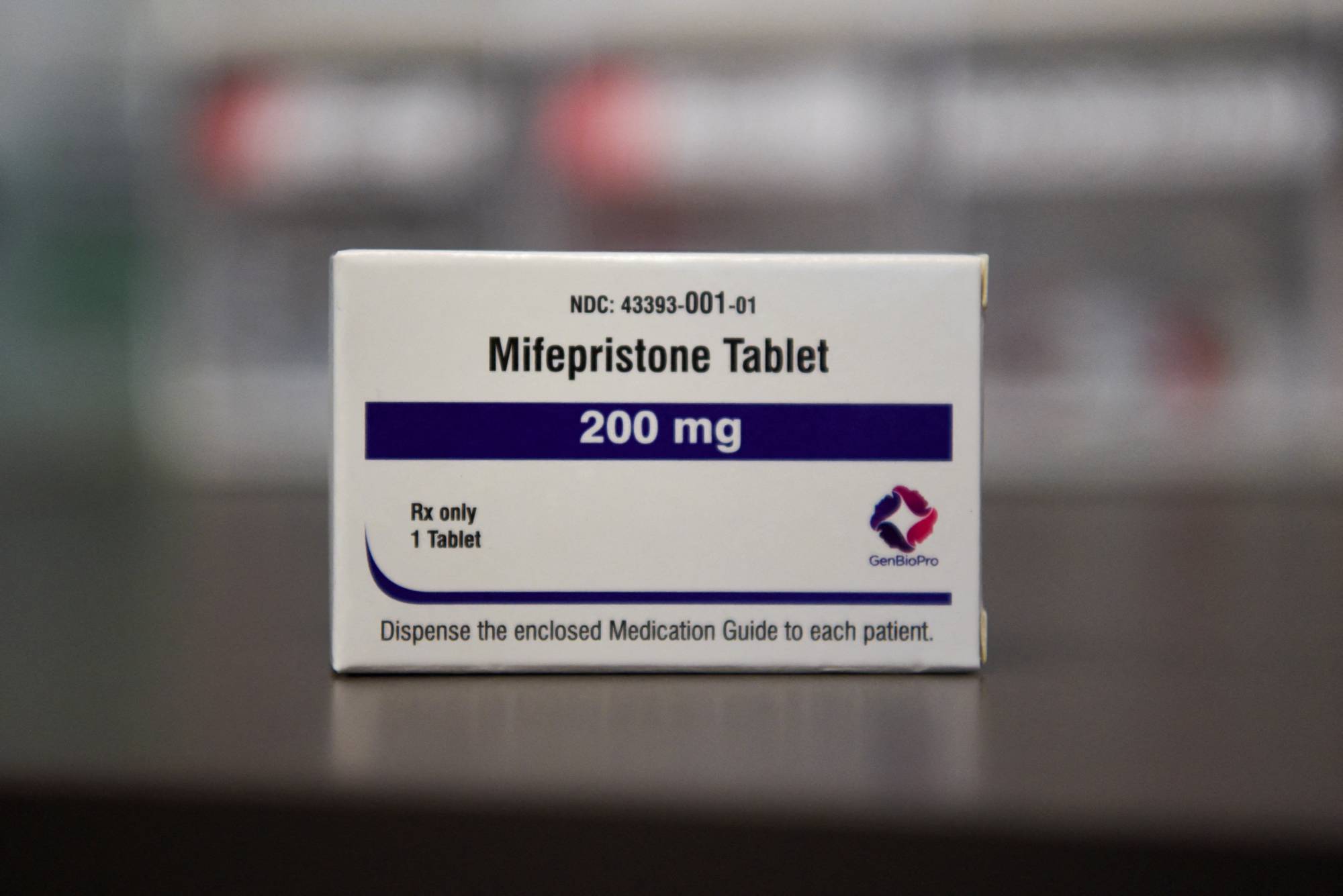
A Texas federal judge’s ruling to remove the 23-year old drug mifepristone from the market not only threatens abortion access in the U.S. — it’s also an appalling sideswipe at the Food and Drug Administration’s authority and expertise.
On Wednesday in the U.S., the Supreme Court was expected to decide on whether to keep the restrictions in place; and if allowed to stand, it will have a stifling effect on pharmaceutical innovation. Discovering and developing a drug is expensive; depending on who you ask, the average cost of bringing a new medicine or vaccine from idea to market is anywhere from a few hundred million dollars to more than $2 billion.
Companies make that investment under the assumption that their products can be sold anywhere in the U.S. for a certain number of years — that they will come out on the other side of the FDA’s rigorous review process with a product that doctors consider safe and effective enough to prescribe, patients trust enough to take and insurance companies regard as worth paying for. They know that if an issue arises with their product, the FDA has a well-defined (albeit far from perfect) process for removing it from the market. The agency’s stamp has become the gold standard for regulatory authorities around the world.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.