As with many other things, the COVID-19 pandemic has delayed the blockbuster fraud trial of Elizabeth Holmes, whose start-up Theranos Inc. went from darling to dumpster fire in a matter of months after the Wall Street Journal exposed huge flaws in the company’s promise its blood-test kits could detect an array of illnesses from a single "finger-prick” of blood. (Holmes has denied the charges.)
Ironically, the pandemic may be recreating a similar environment to the one that cultivated Theranos in the first place, as whistleblower Tyler Shultz, a former employee at the now-defunct start-up, warned on May 2. Pointing to a combination of large-scale health needs, the potential for lucrative financial gain and corner cutting by regulators, he concluded that Theranos would be "thriving” in this pandemic. And when it comes to COVID-19 antibody tests, he has a point.
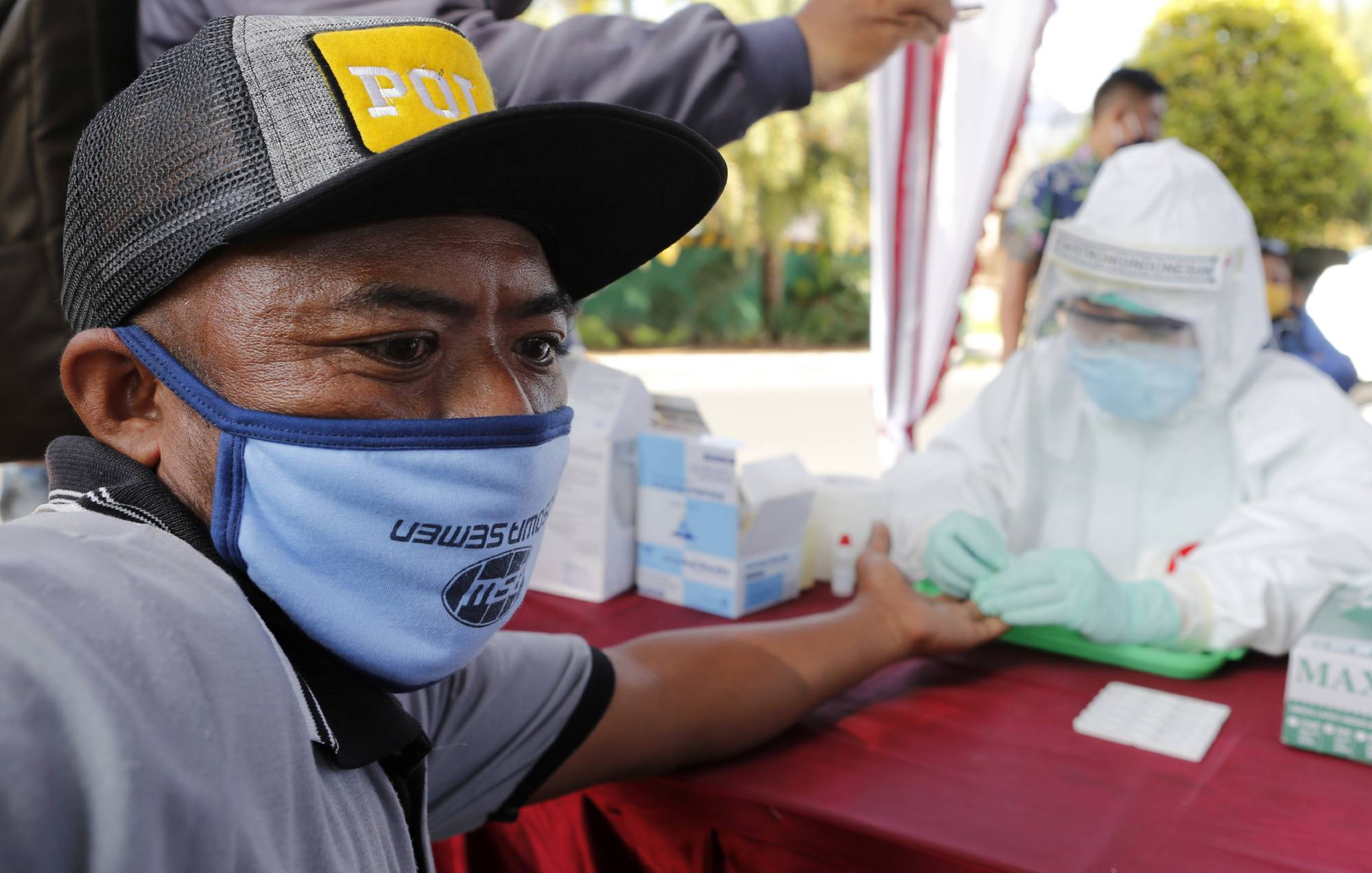
A deluge of new rapid test kits has offered a seductive vision: A single finger-prick of blood is all it might take to see if you’ve been infected with the novel coronavirus in the past and generated antibodies that protect against future reinfection (though this is a topic of debate).


















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.