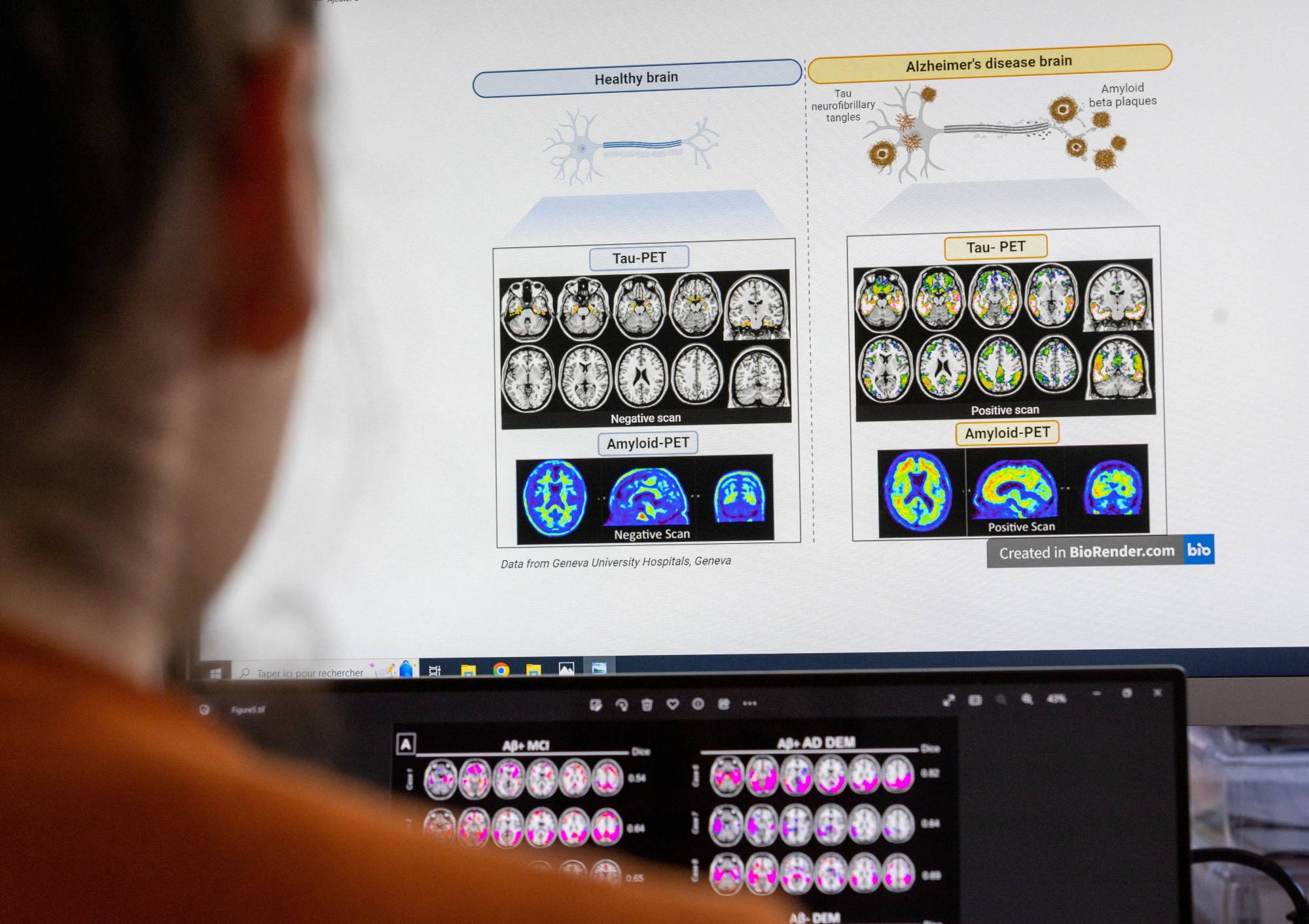
Alzheimer's disease experts in Europe weighing potential use of a new drug from Eisai and Biogen say its ability to slow cognitive decline may not outweigh its health risks, or be worth the toll on scarce health care resources.
Lecanemab, branded Leqembi in the U.S., is under regulatory review in Europe and likely poised for traditional U.S. approval next month based on trial data showing it slowed cognitive decline by 27% in patients with early Alzheimer's.
In Europe, where cost-conscious countries rigorously weigh new drugs before adopting their use, nine neurologists and researchers across six countries told Reuters lecanemab is unlikely to be widely used if approved. Their views underpin analyst estimates suggesting Europe will be a small market for the drug.


















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.