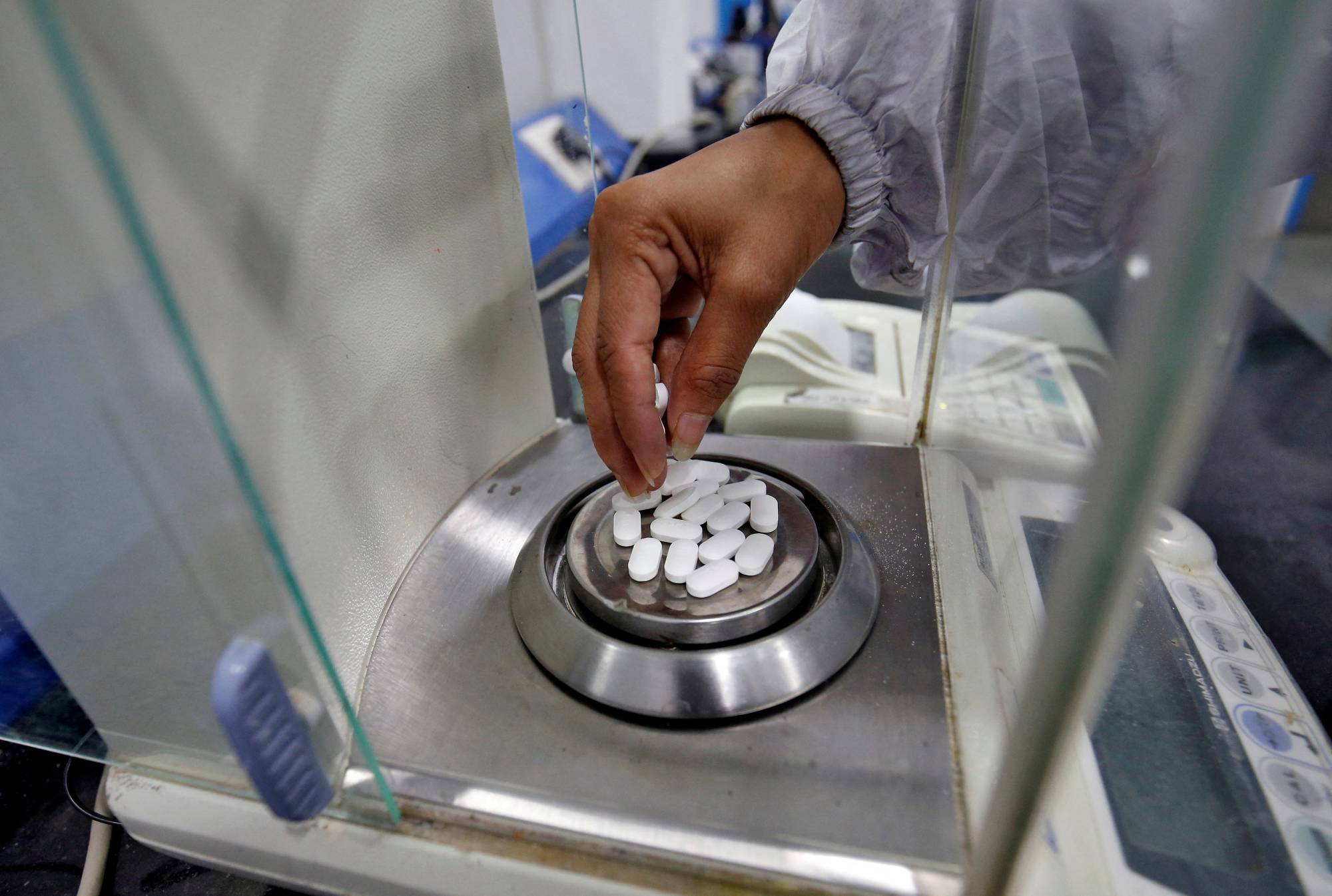
U.S. inspectors have in recent months uncovered wide-ranging lapses at factories run by some of India’s biggest pharmaceutical firms, as the world’s top supplier of cheap medicine faces increased scrutiny after a spate of deadly manufacturing incidents.
Dozens of drugmakers were issued notices and warning letters by the U.S. Food and Drug Administration, which is increasing visits to Indian factories after the lifting of COVID-19 restrictions last year. Inspectors detailed unsanitary conditions in manufacturing plants and poorly trained staff, shredded paperwork and customer complaints that were not properly followed up on and evidence of exporting contaminated drugs to the U.S.
The expansive lapses — detailed in FDA records obtained under the Freedom of Information Act (FOIA) — suggest that a nearly two-year hiatus in factory audits during the pandemic meant faults were missed in some Indian plants that export to the U.S.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.