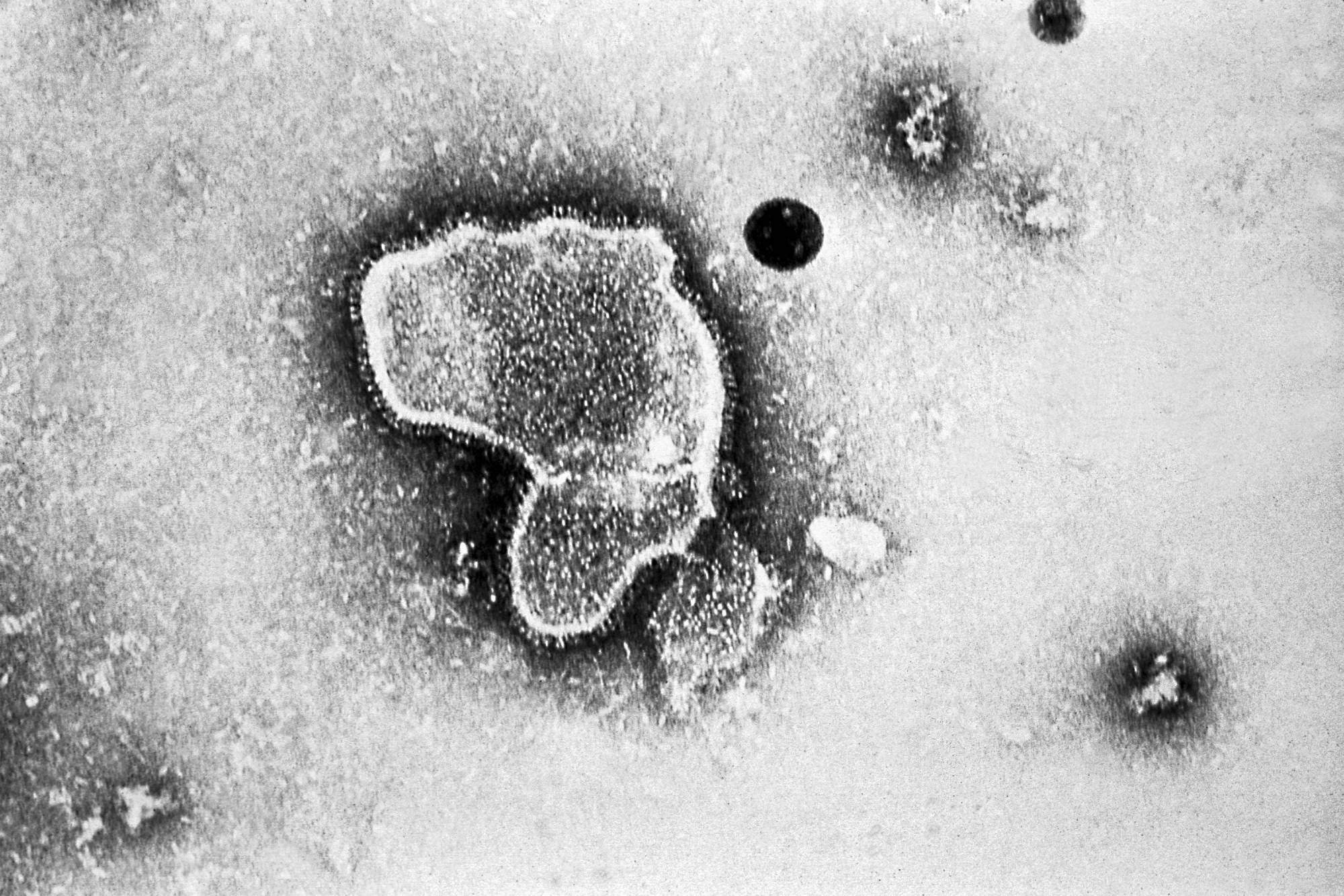
For 60 years, doctors and scientists searched for a vaccine against a common virus that, while sometimes deadly, is little known to the public. The hunt is over.
The U.S. Food and Drug Administration cleared GSK’s shot against respiratory syncytial virus on Wednesday. The product will go on sale in the coming weeks for older adults.
Medical breakthroughs rarely come alone, and this one is no exception. GSK, seeking to reassert its role as a key vaccine industry player after falling behind in COVID-19, will likely be forced to battle it out with one of the biggest pandemic winners, Pfizer, within weeks.


















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.