China is in talks with Pfizer Inc. to secure a license that will allow domestic drugmakers to manufacture and distribute a generic version of the U.S. firm's COVID-19 antiviral drug Paxlovid in China, three sources have said.
China's medical products regulator — the National Medical Products Administration (NMPA) — has been leading the talks with Pfizer since late last month, one of the sources with knowledge of the matter said.
Beijing is keen to finalize licensing deal terms before the Lunar New Year which begins on Jan. 22, the source said.



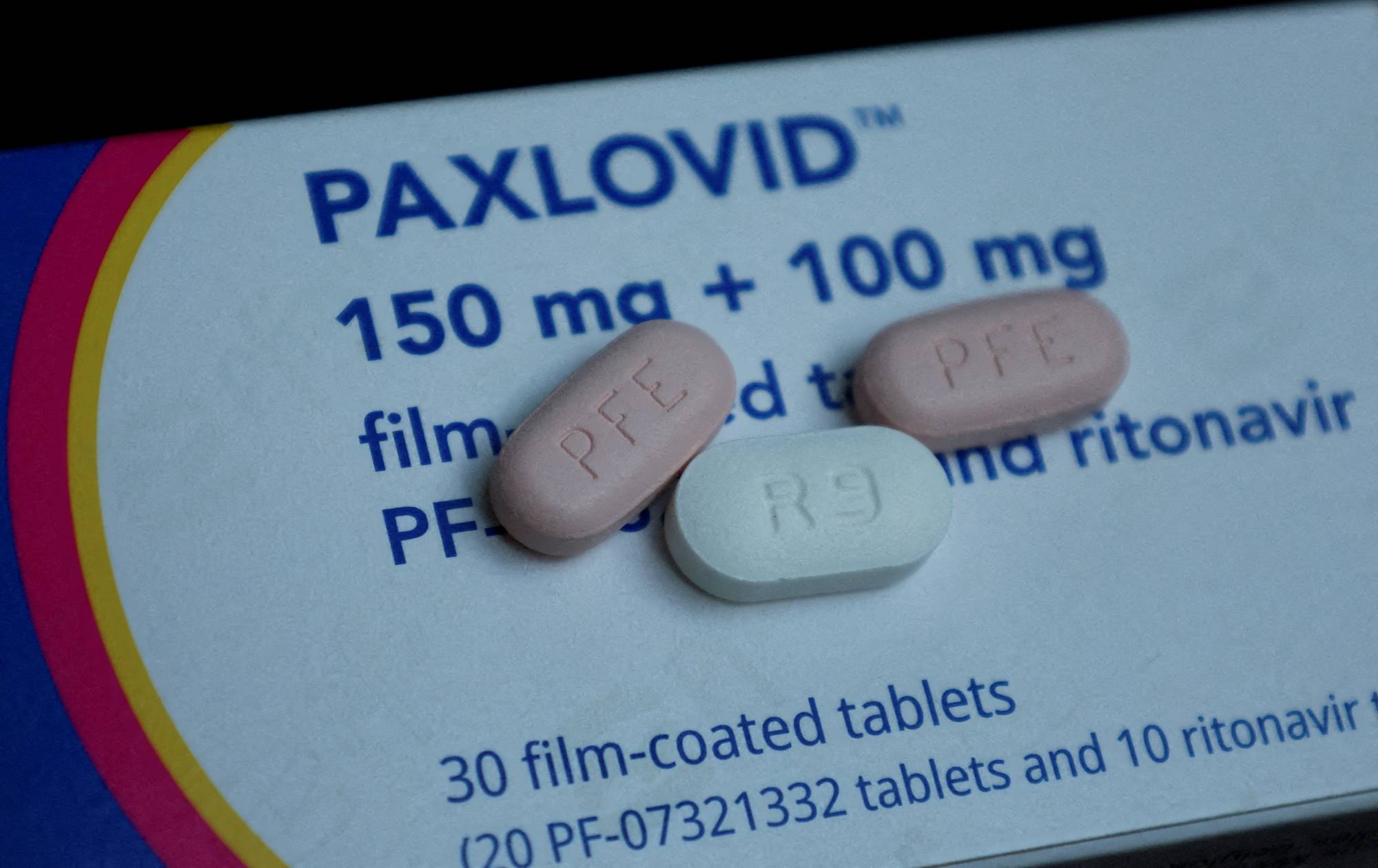














With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.