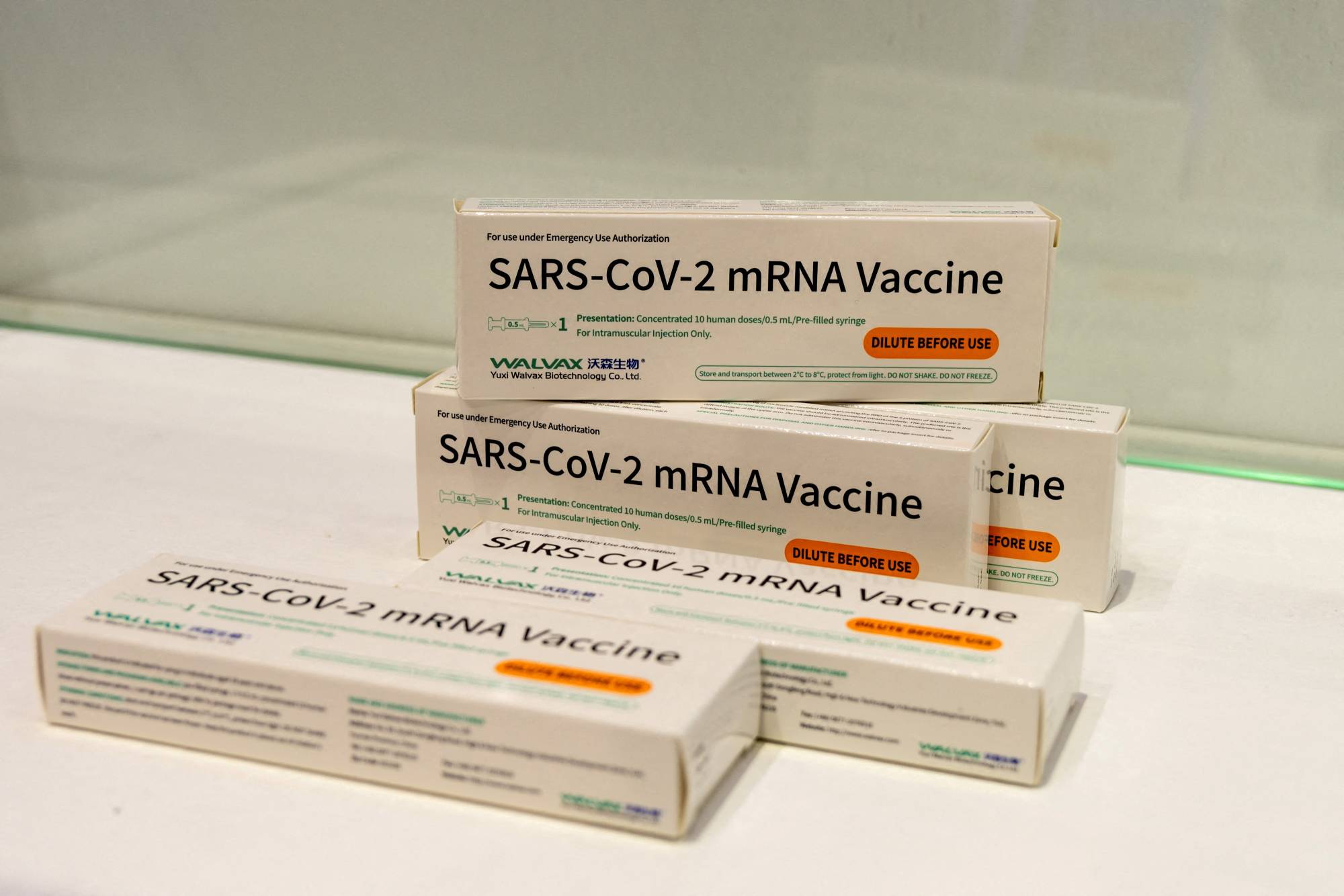
Indonesia said it has granted approval for emergency use to an mRNA COVID-19 vaccine developed by a Chinese company, becoming the first country, ahead of even China, to do so.
Indonesia's food and drugs agency (BPOM) greenlighted the use of Walvax Biotechnology's mRNA vaccine, which has been in development for more than two years and targets the original strain of the coronavirus.
The approval comes as a surprise as Walvax, which has been conducting large late-stage trials of the vaccine in several countries including Indonesia, Mexico and China, has yet to publish efficacy readings that would show how well it can reduce the risk of COVID-19 cases and deaths.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.