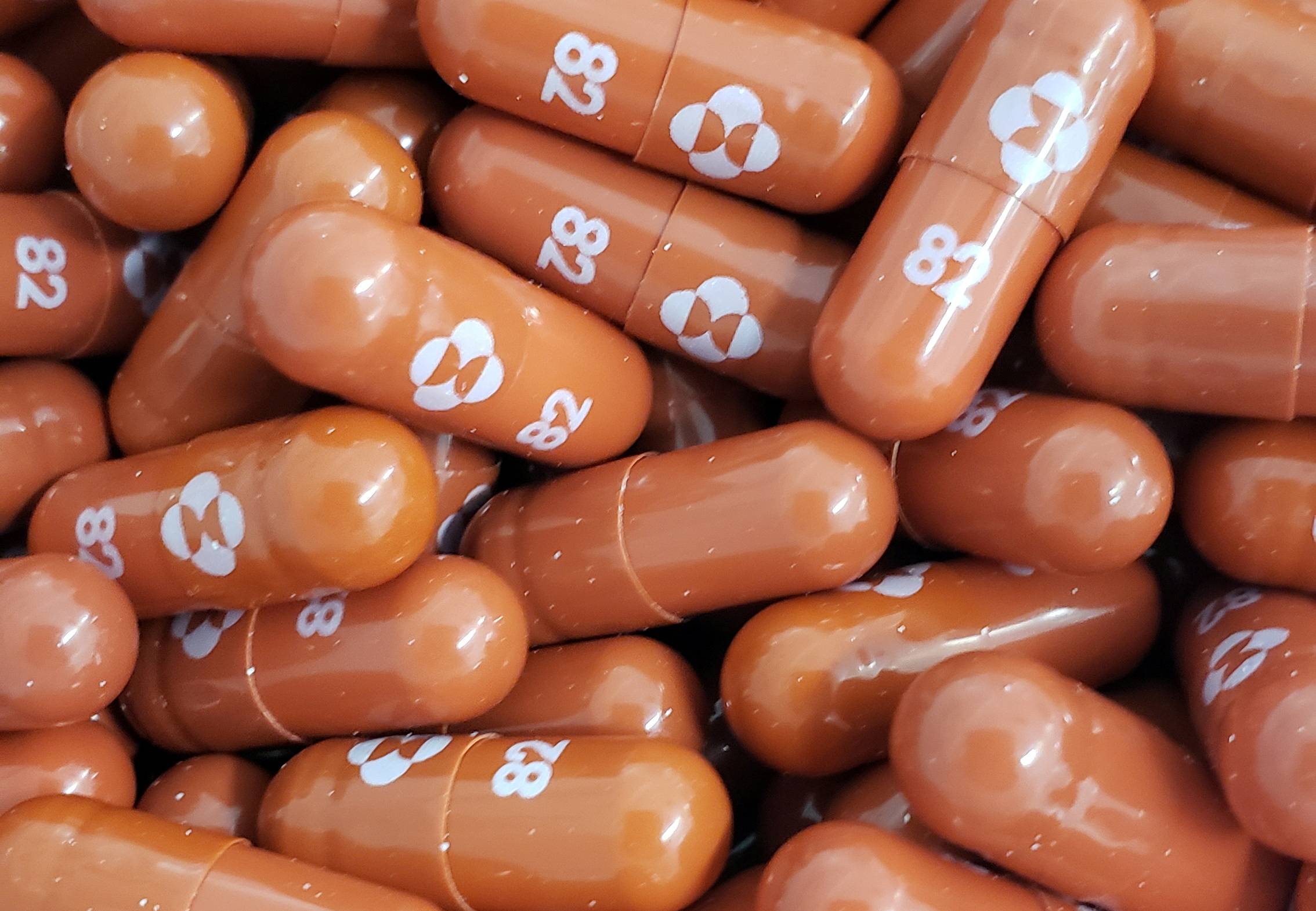
Pharmaceutical company Merck said Friday that in a final analysis of a clinical trial, its antiviral pill reduced the risk of hospitalization and death among high-risk COVID-19 patients by 30%, down from an earlier estimate of 50%.
The lower efficacy is a disappointment for the drug, known as molnupiravir, which health officials around the world are counting on as a critical tool to save lives and reduce the burden on hospitals. It increases the importance of a similar, apparently more effective, offering from Pfizer that is also under review by the U.S. Food and Drug Administration.
A panel of advisers to the FDA is set to meet Tuesday to discuss Merck’s treatment and vote on whether to recommend authorizing it to treat high-risk COVID-19 patients.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.