The government has expressed interest in introducing an oral antiviral drug for COVID-19 by the end of the year, with Prime Minister Fumio Kishida saying that such treatments could become a “key trump card” in the prolonged, uphill battle against the coronavirus.
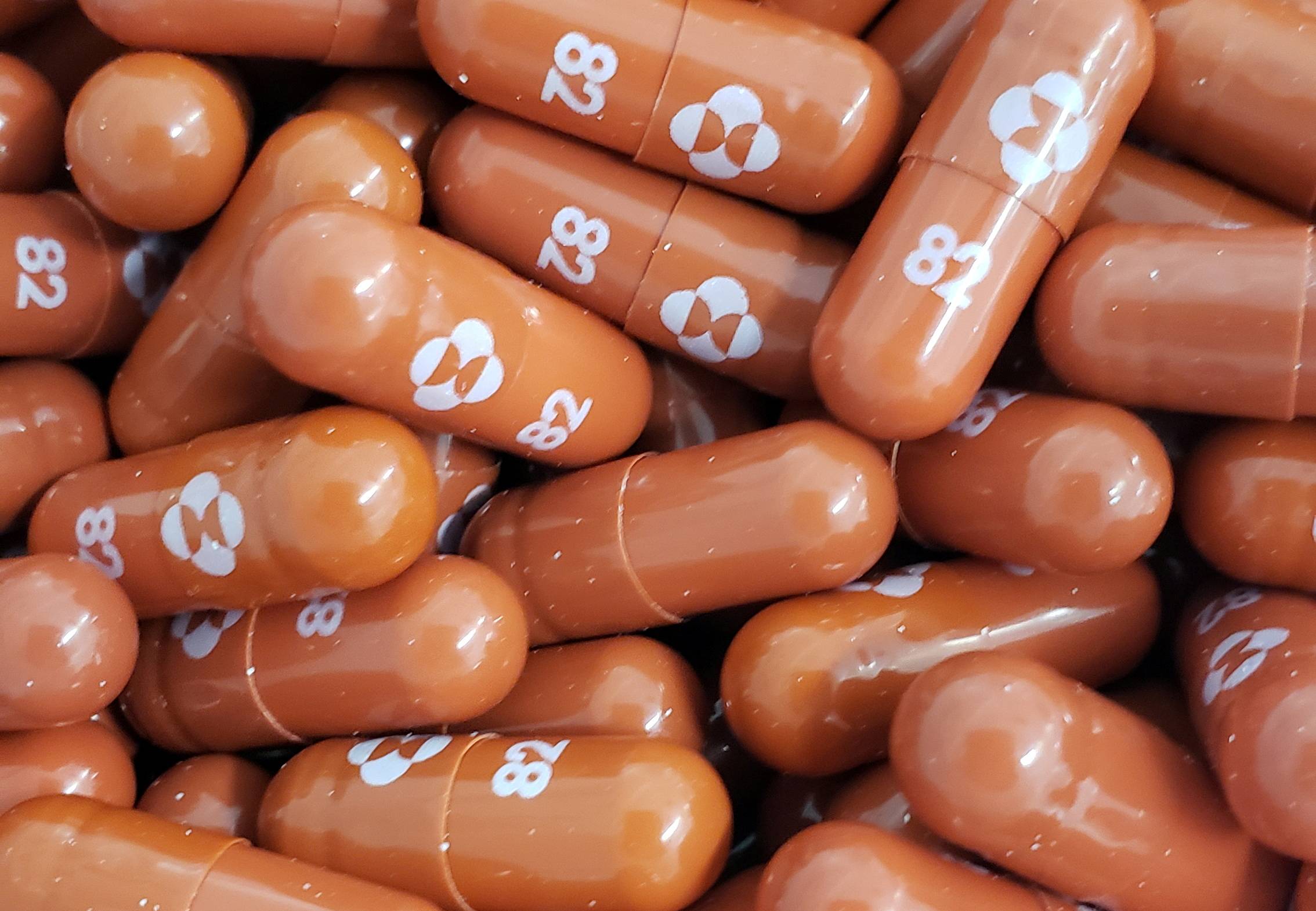
U.S. drugmaker Merck & Co. and Miami-based Ridgeback Biotherapeutics last week filed an emergency use authorization application with the U.S. Food and Drug Administration for molnupiravir, for the treatment of mild and moderate COVID-19 symptoms in adults who are at risk of hospitalization or developing a severe condition. The FDA is set to meet on Nov. 30 to weigh Merck's request.
Merck’s Japan unit is expected to file for fast-track approval in the country soon. Once the FDA gives its authorization — which could come by the end of the year — the Japanese government, which has been in talks to buy the drug, is expected to give approval for domestic use, something that could follow soon after.


















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.