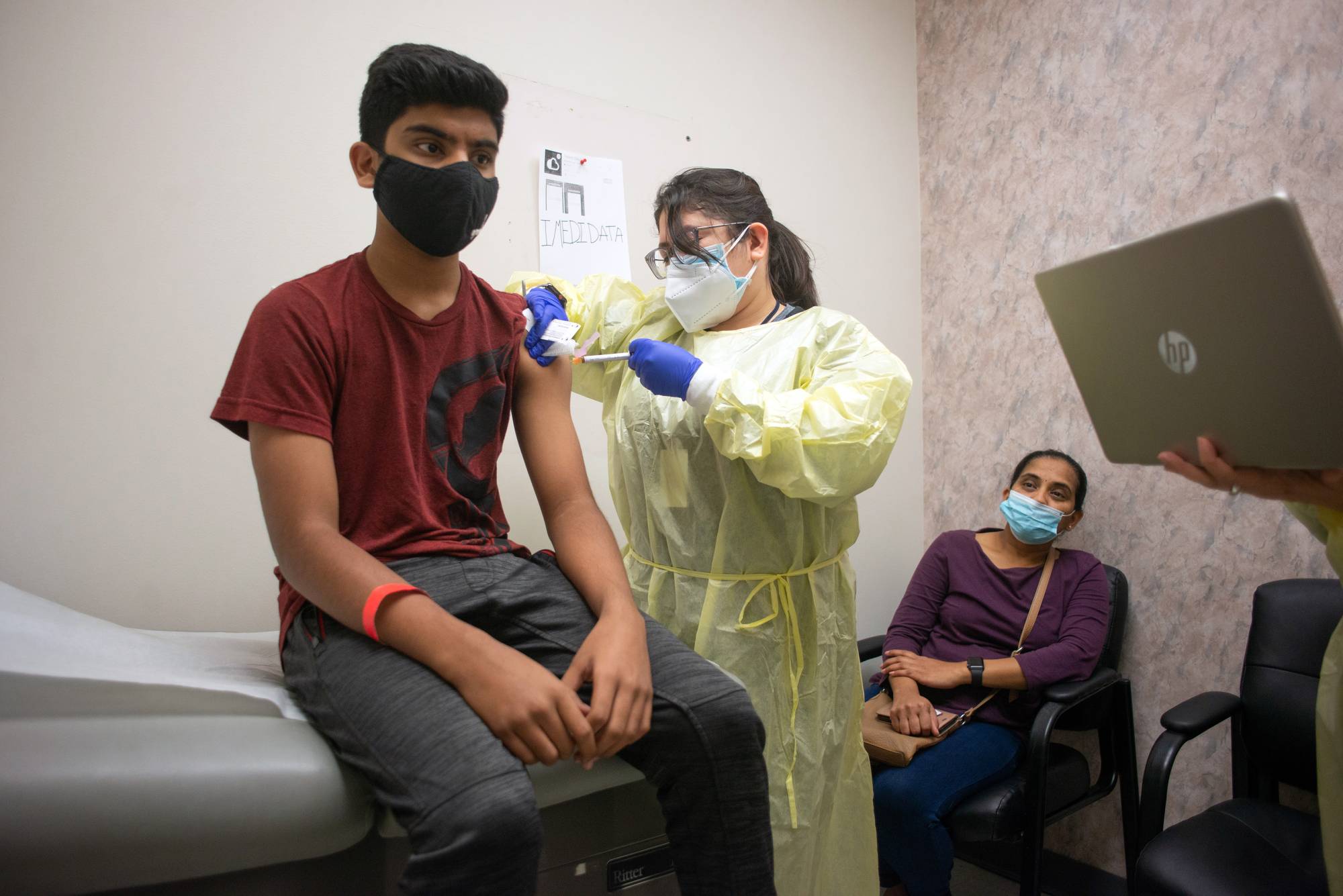
Moderna Inc.’s COVID-19 vaccine was highly effective in 12- to 17-year-old adolescents in a large study, paving the way for regulatory submissions around the world by early June.
In a news release, the company said its vaccine was between 93% and 100% effective in preventing symptomatic COVID-19 in a study of teenagers, depending on whether very mild cases are included in the count. The study met its primary goal of showing that immune responses to the vaccine in 12- to 17-year-olds were as good as those produced in adults, and no significant safety concerns were observed, according to the company.
The results put Moderna’s vaccine, currently authorized in the U.S. for people 18 and up, on track to soon become the second shot authorized in the U.S. for younger teens. Earlier this month, the U.S. Food and Drug Administration expanded clearance of the Pfizer Inc.-BioNTech SE vaccine to include teenagers ages 12 to 15. That vaccine was originally authorized for those 16 and up.


















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.