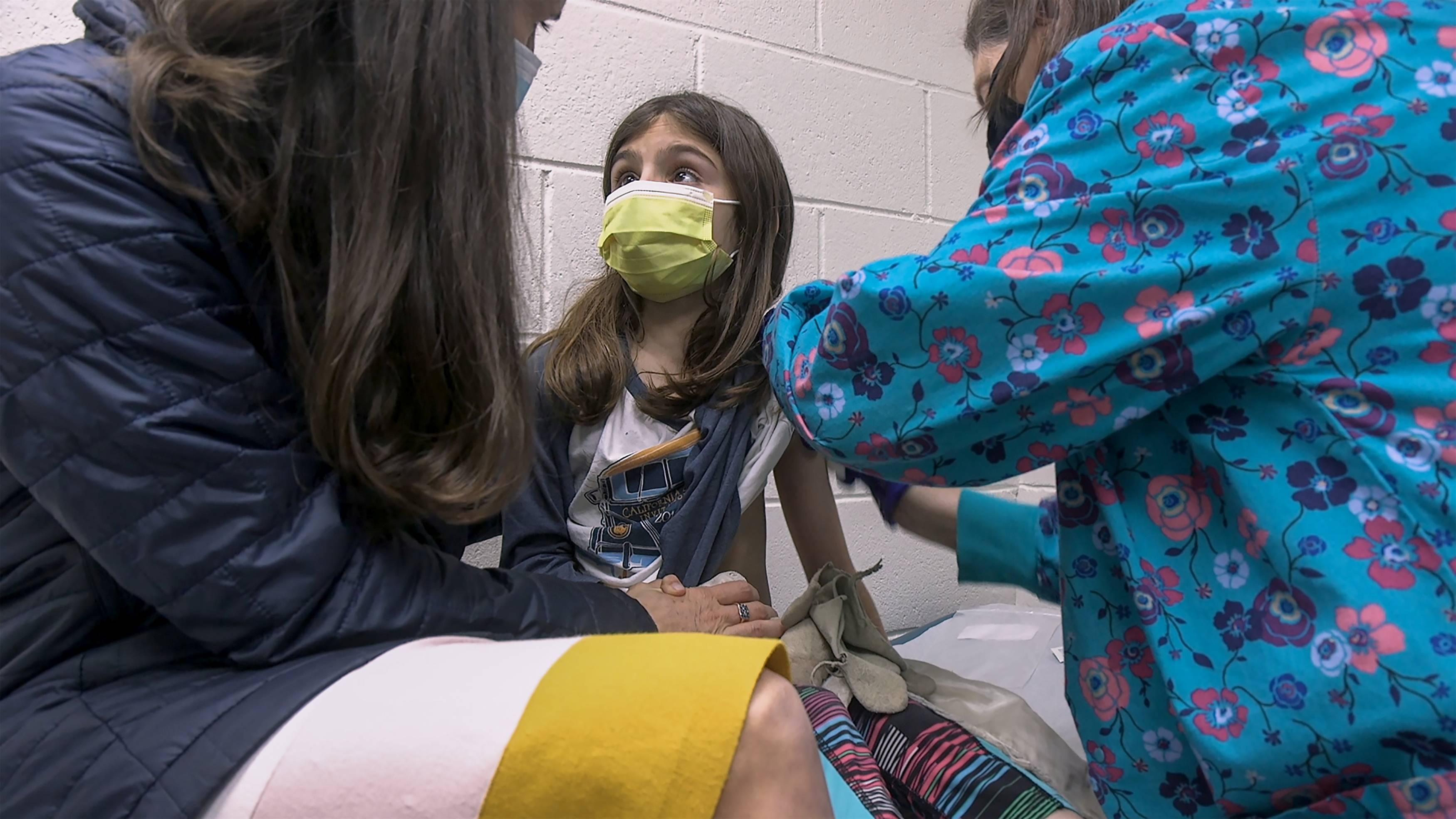
Pfizer Inc. and BioNTech SE’s COVID-19 vaccine has been cleared for use in younger teens in the U.S., paving the way for the mass vaccination of middle- and high-school students before the start of summer camps and the next school year.
The Food and Drug Administration said in a statement Monday that it had expanded the shot’s original emergency use authorization to include adolescents age 12 to 15.
"It was a relatively straightforward decision,” said Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, at a news conference. The vaccine generated a strong immune response, there were no cases of COVID-19 in the vaccinated group, and there were no signs of any new or worrisome side effects, he said.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.