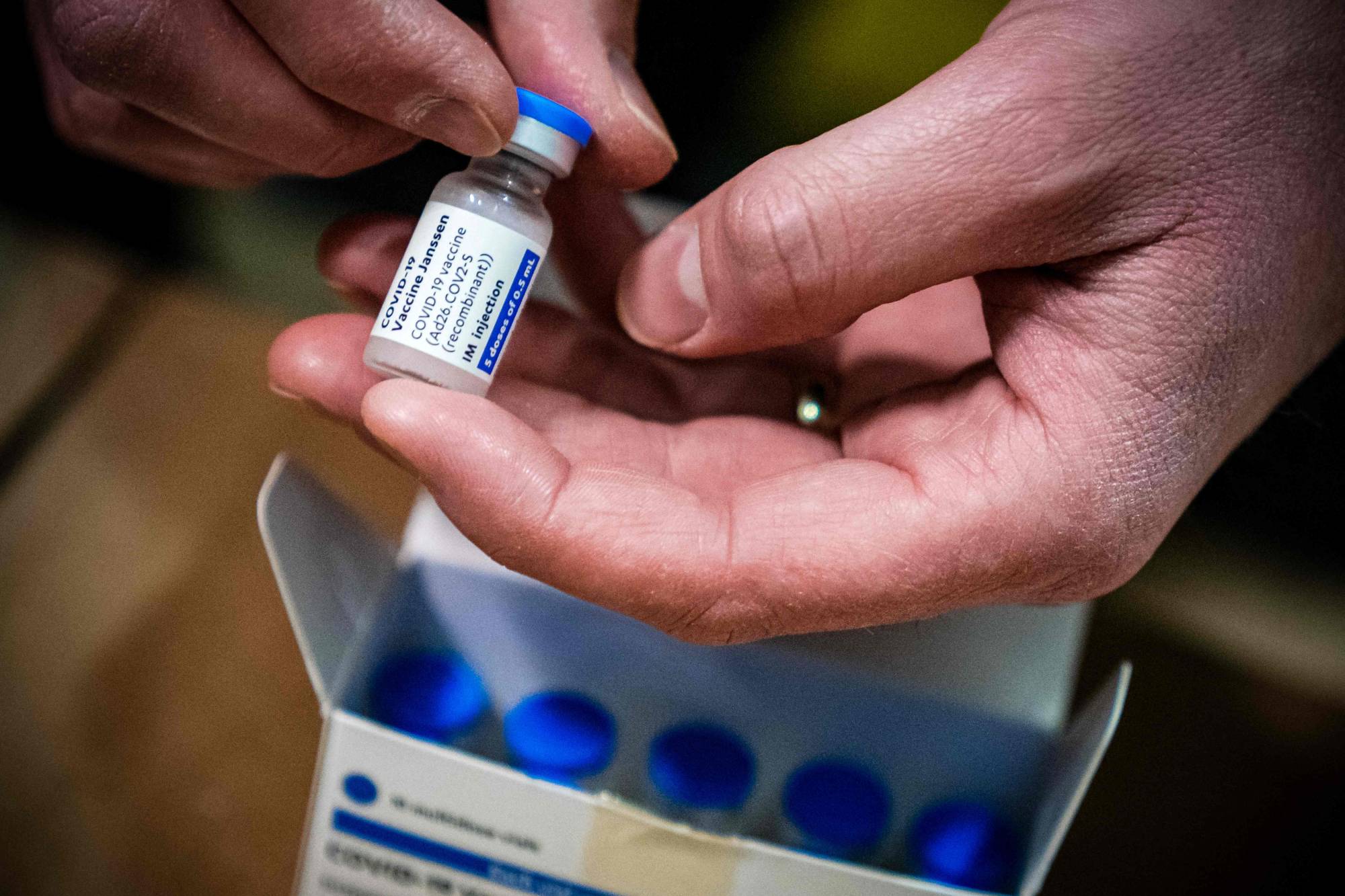
U.S. federal health agencies on Tuesday will call for an immediate pause in use of Johnson & Johnson’s single-dose coronavirus vaccine after six recipients in the United States developed a rare disorder involving blood clots within about two weeks of vaccination, officials briefed on the decision said.
All six recipients were women between the ages of 18 and 48. One woman died and a second woman in Nebraska has been hospitalized in critical condition, the officials said.
Nearly 7 million people in the United States have received Johnson & Johnson shots so far, and roughly 9 million more doses have been shipped out to the states, according to data from the Centers for Disease Control and Prevention (CDC).

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.