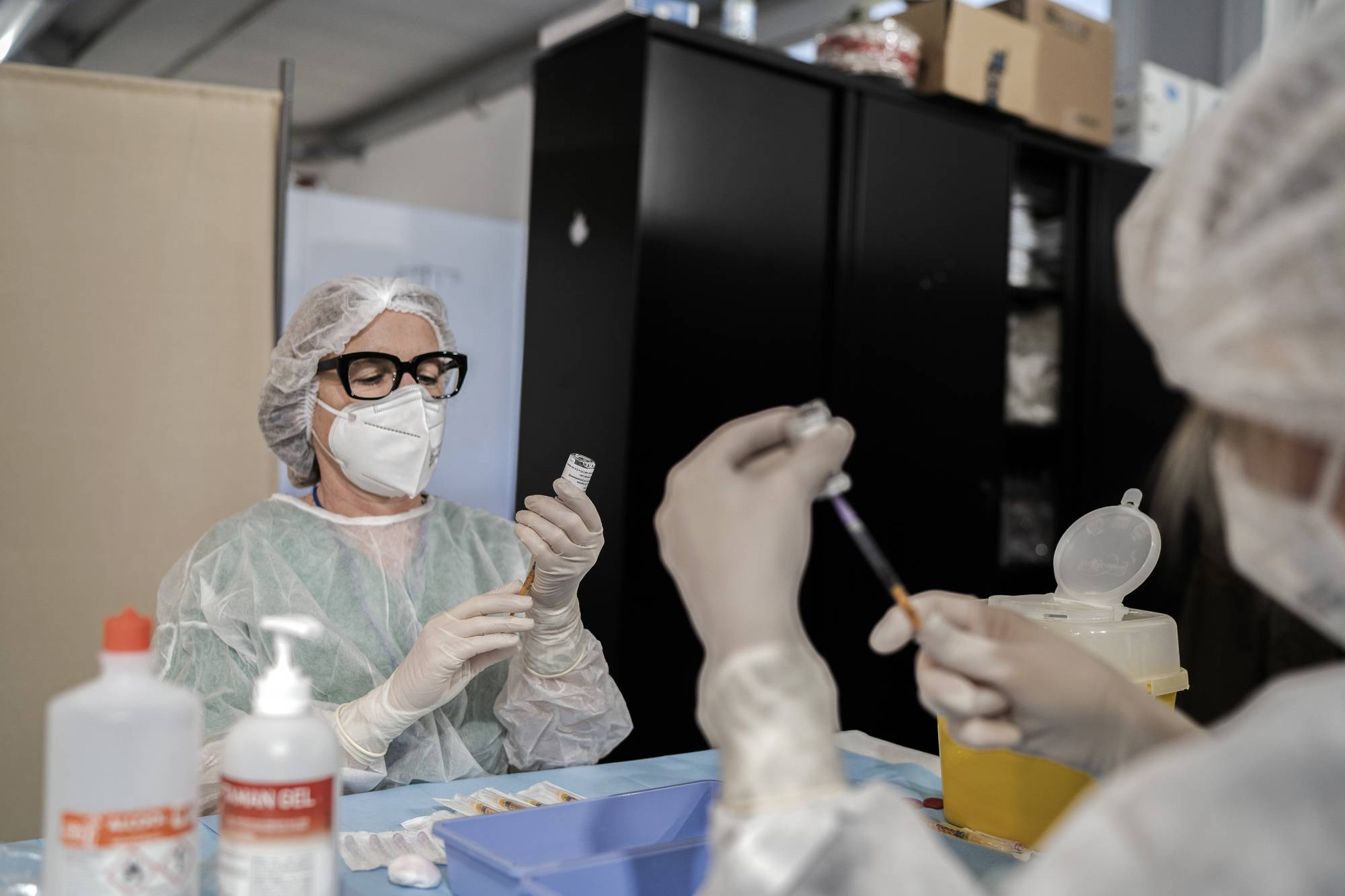
AstraZeneca's COVID-19 vaccine performed better than expected in a major late-stage trial, paving the way for its potential emergency authorization in the United States and boosting confidence in the shot after setbacks in Europe.
The drugmaker said on Monday that interim data from trials in Chile, Peru and the United States found the vaccine was 79% effective in preventing symptomatic COVID-19 and, crucially, posed no increased risk of blood clots.
AstraZeneca intends to request U.S. emergency authorization for the vaccine, which was developed in conjunction with Oxford University, in the coming weeks.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.