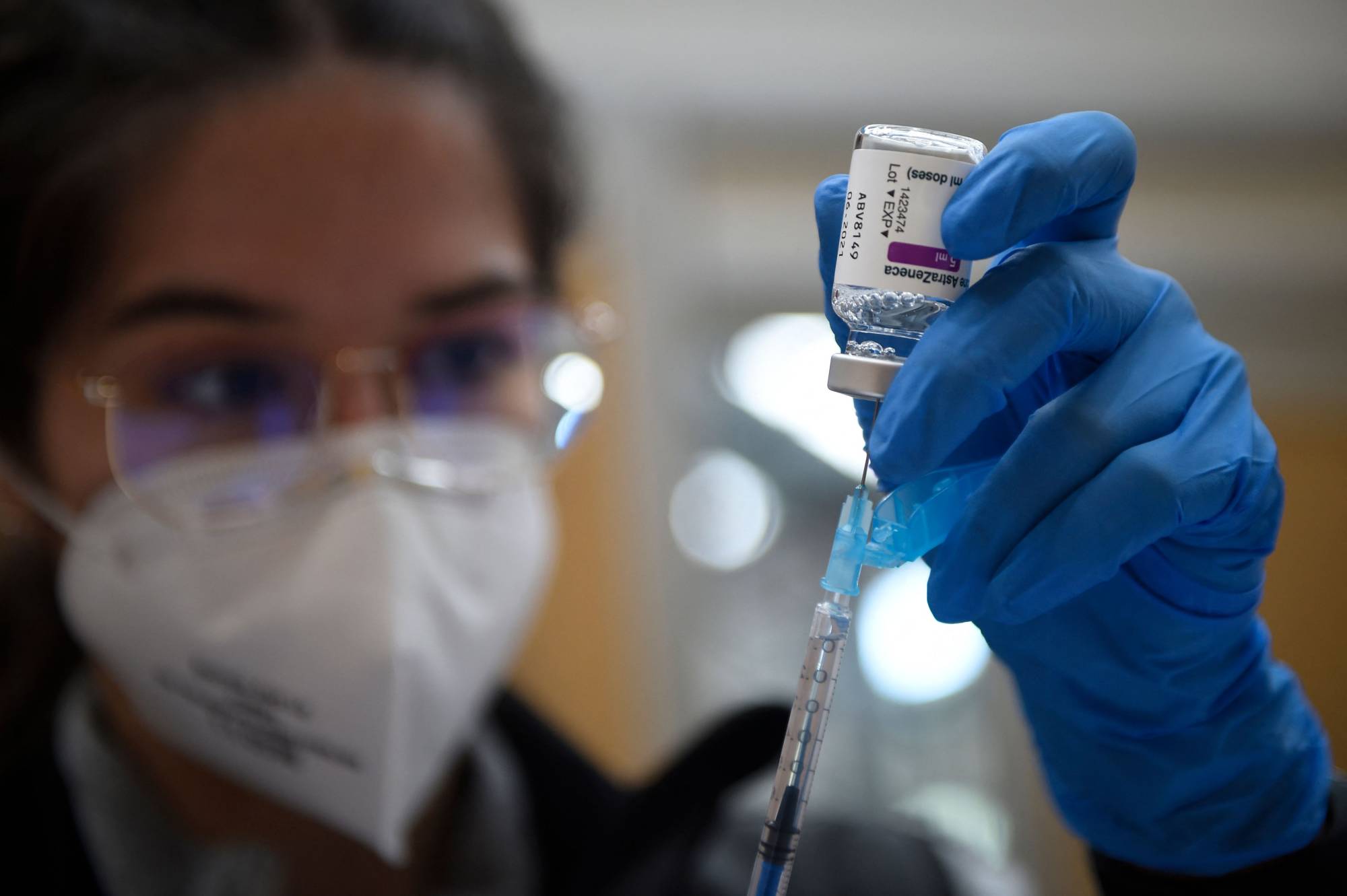
The EU's drug watchdog said on Thursday it is still convinced the benefits of AstraZeneca's COVID-19 vaccine outweigh the risks following an investigation into reports of blood clots that prompted more than a dozen nations to suspend its use.
European Medicines Agency (EMA) director Emer Cooke said the watchdog could not definitively rule out a link between blood clot incidents and the vaccine in its investigation into 30 cases of a rare blood clotting condition.
But she said the "clear" conclusion of the review was that the benefits in protecting people from the risk of death or hospitalization outweighs the possible risks. The issue deserves further analysis, the EMA said.


















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.