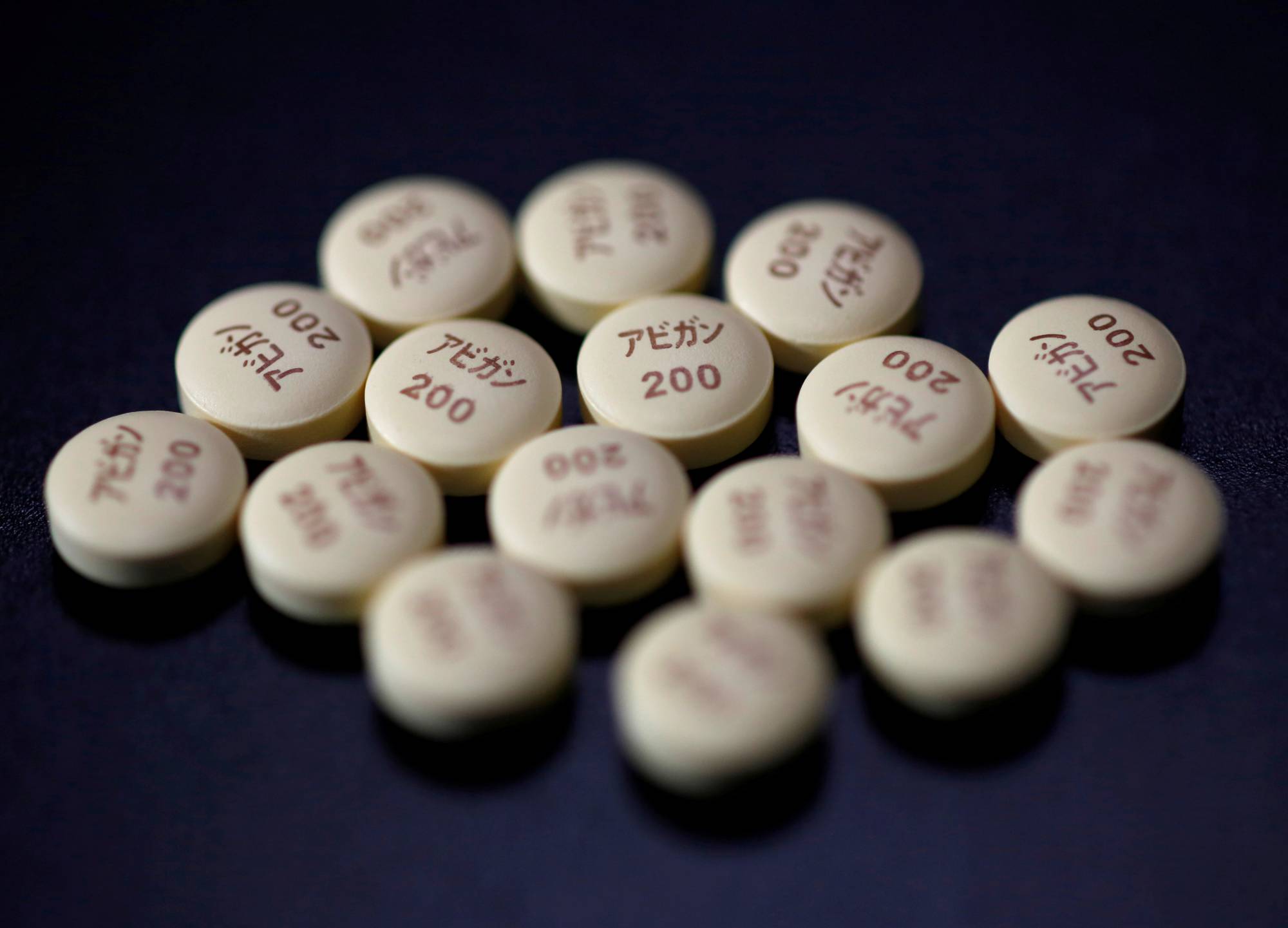
Fujifilm Holdings Corp will restart a clinical trial in Japan of its antiviral drug Avigan for the treatment of COVID-19, the Nikkei newspaper reported on Sunday.
Domestic approval of the drug was delayed after a health ministry panel said in December that trial data was inconclusive. The new study will involve about 270 patients and Fujifilm will aim to seek approval again in October, Nikkei said.
Representatives from Fujifilm did not immediately respond to a request for comment.
Japan has approved Avigan, known generically as favipiravir, as an emergency flu medicine. But concerns remain, as the drug has been shown to cause birth defects in animal studies and its effectiveness against COVID-19 has proven difficult to demonstrate.
Japan's government has called on Fujifilm to triple national stockpiles of the drug, which has been approved for COVID-19 treatment in Russia, India and Indonesia.


















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.