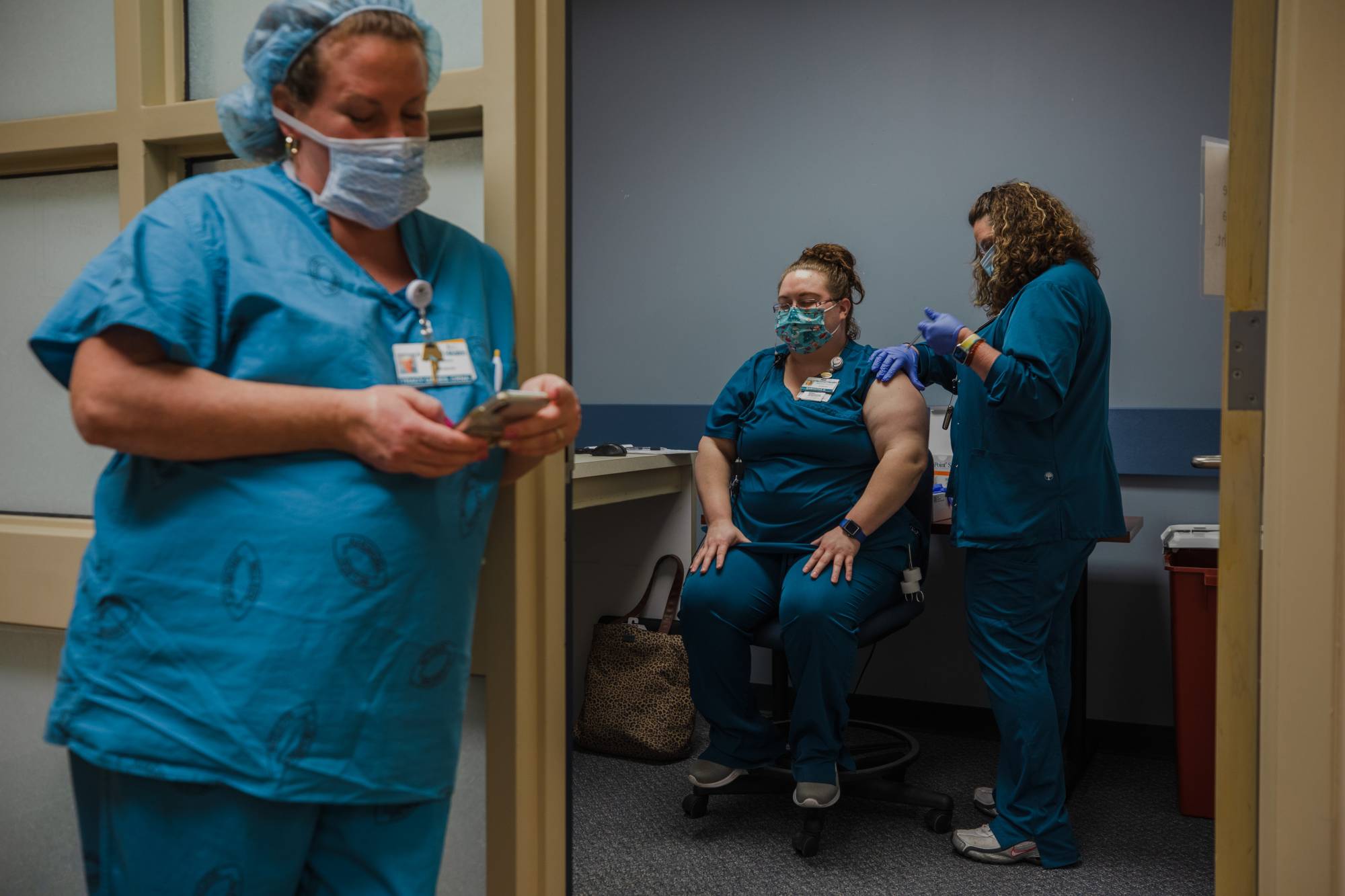
Major U.S. drugmaker Pfizer Inc. on Friday filed for a fast-track approval in Japan of its COVID-19 vaccine, marking the first such application made in the nation, with industry sources saying the formal approval could come within months amid the ongoing novel coronavirus pandemic.
The U.K. and the U.S. have already started this month administering the firm's mRNA vaccine, developed jointly with Germany’s BioNTech SE, and this week Singapore became the first Asian country to grant approval for its use.
Friday’s application came about two months after Pfizer started the first two phases of the drug's three-phase clinical trial in Japan, conducted on 160 Japanese age 20 to 85.















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.