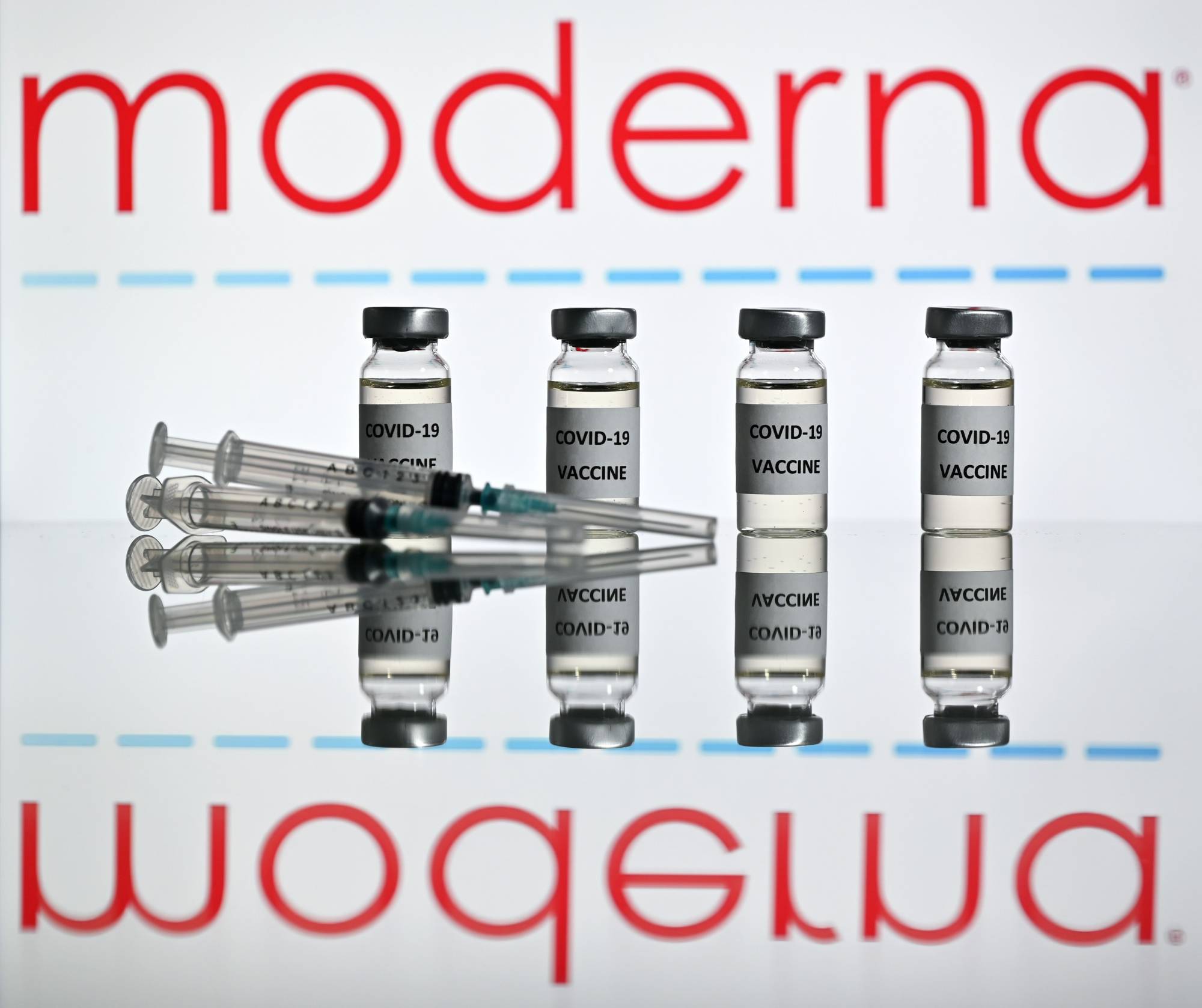
Moderna Inc. plans to request clearance for its coronavirus vaccine in the U.S. and Europe on Monday, after a new analysis showed the vaccine was highly effective in preventing COVID-19 with no serious safety problems.
The primary analysis, which included 196 cases, found the vaccine was 94.1% effective, in line with preliminary findings released earlier this month. None of the participants in the trial who had received the vaccine developed severe COVID-19. All 30 severe cases observed in the study occurred in participants who received placebo shots, according to a company statement.
The new results put the Cambridge, Massachusetts-based biotechnology company on track to have one of the first COVID-19 vaccines to be cleared in the U.S. A similar vaccine from Pfizer Inc. and BioNTech SE was submitted to U.S. regulators earlier this month and is scheduled to be reviewed ahead of Moderna’s shot.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.