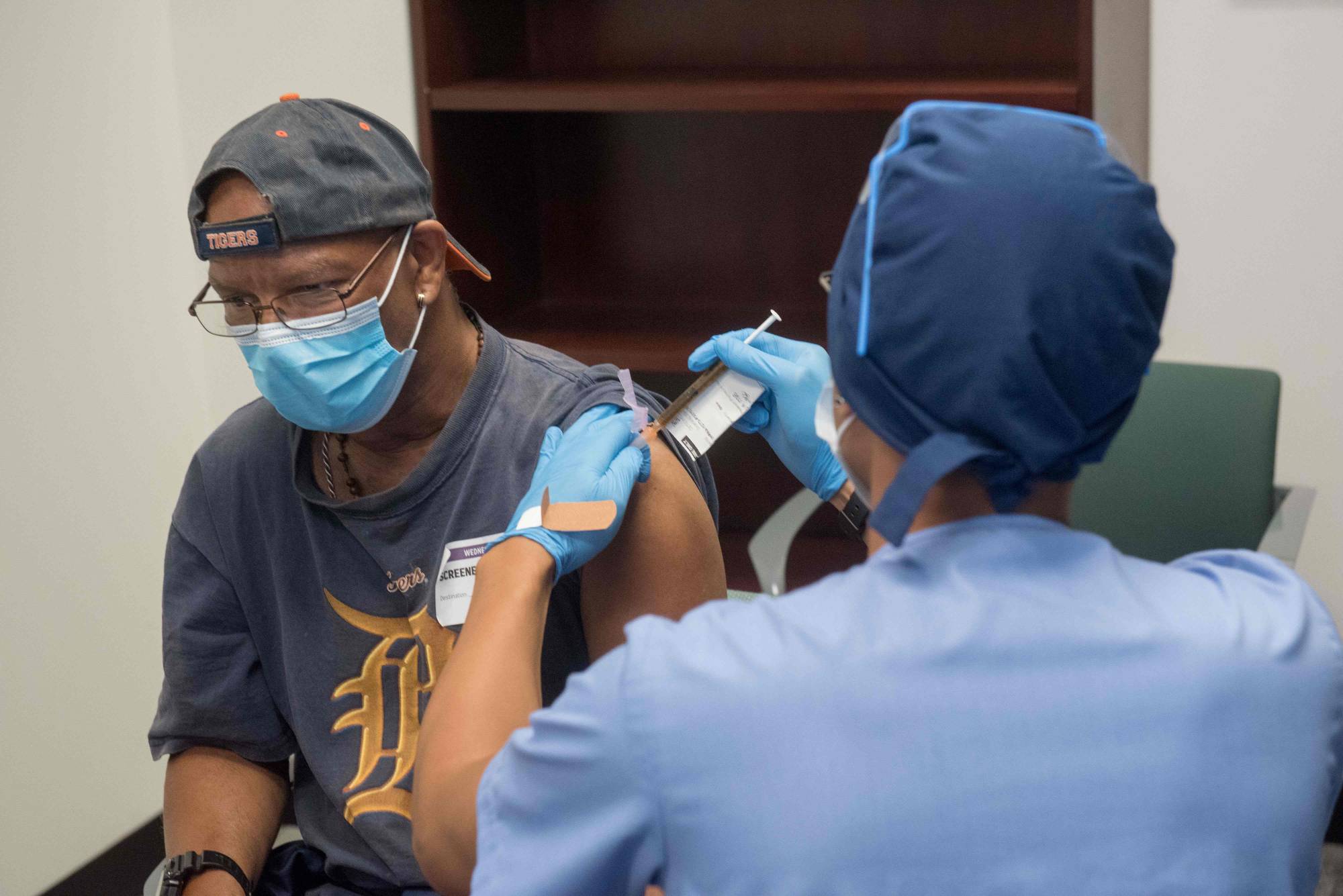
Now it’s Moderna Inc.’s time to be in the spotlight.
The same U.S. explosion of COVID-19 cases that helped Pfizer Inc. get results for its vaccine trial earlier this week is helping speed along Moderna’s trial. Moderna said Wednesday its study has accumulated more than 53 infections, allowing a preliminary analysis of the shot’s effectiveness to begin. The shares jumped.
Moderna didn’t predict how long it could take an independent monitoring committee to analyze the data, but said the company could get the data to the committee within days. The company said it is still blinded to the data.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.