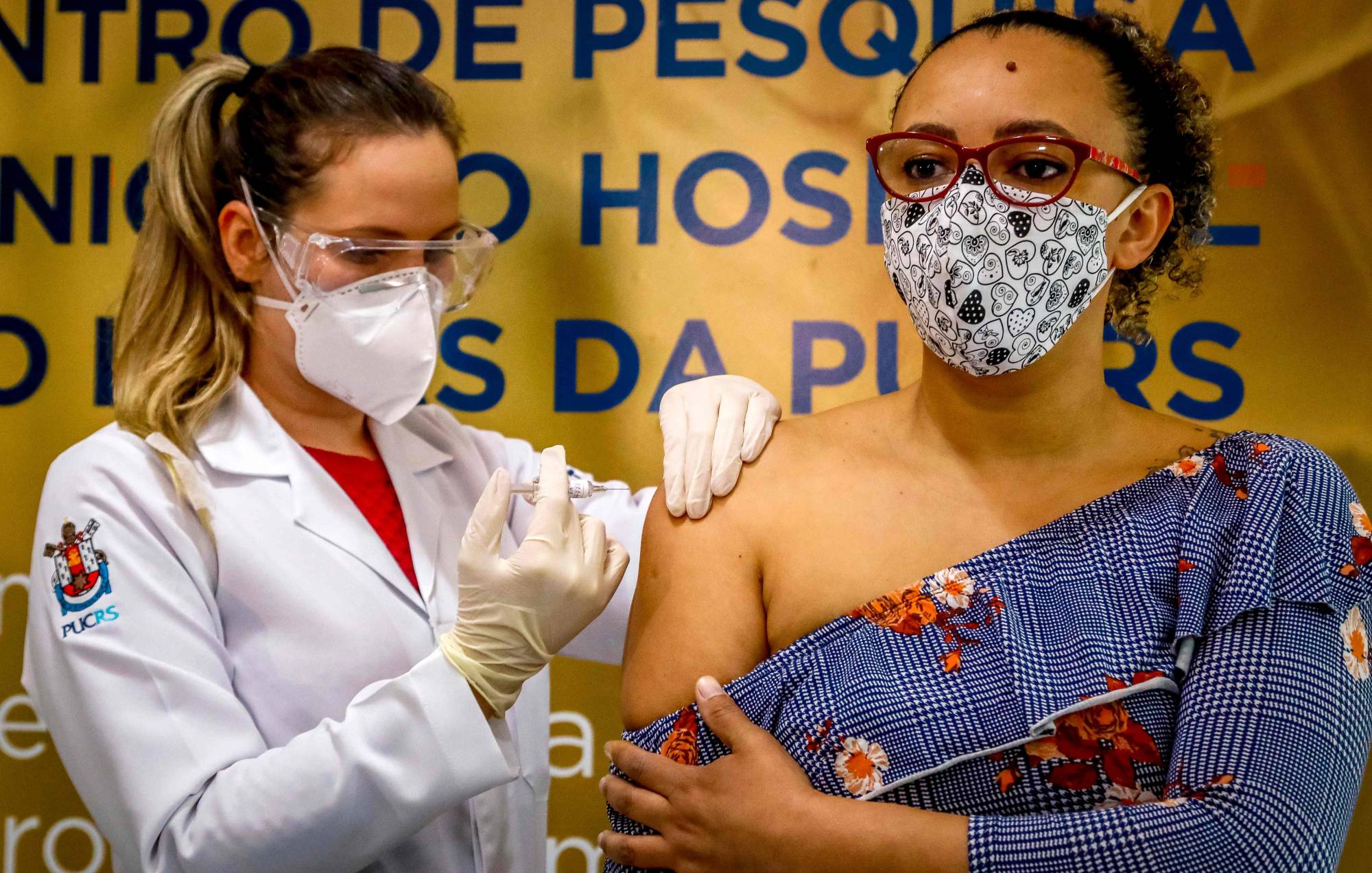
The final-stage trial of a Chinese front-runner vaccine candidate has been halted in Brazil due to a serious adverse event, the first time that any of the Asian nation’s rapidly developed COVID-19 shots have met with such a setback.
Testing of Sinovac Biotech Ltd.’s vaccine, called Coronavac, has been halted in Brazil after an event that occurred on Oct. 29, said the Brazil Health Agency on Tuesday, without giving any further detail on what happened. The study is interrupted in accordance with regulations while the agency analyzes if the study should continue, it said.
Sao Paulo’s Instituto Butantan, which partnered with Sinovac to produce the vaccine locally, said in a statement it was surprised by the decision and is looking into details of what happened in the study. Director Dimas Covas said in a TV interview that one volunteer in the trial has died, but that the death is not related to the vaccine.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.