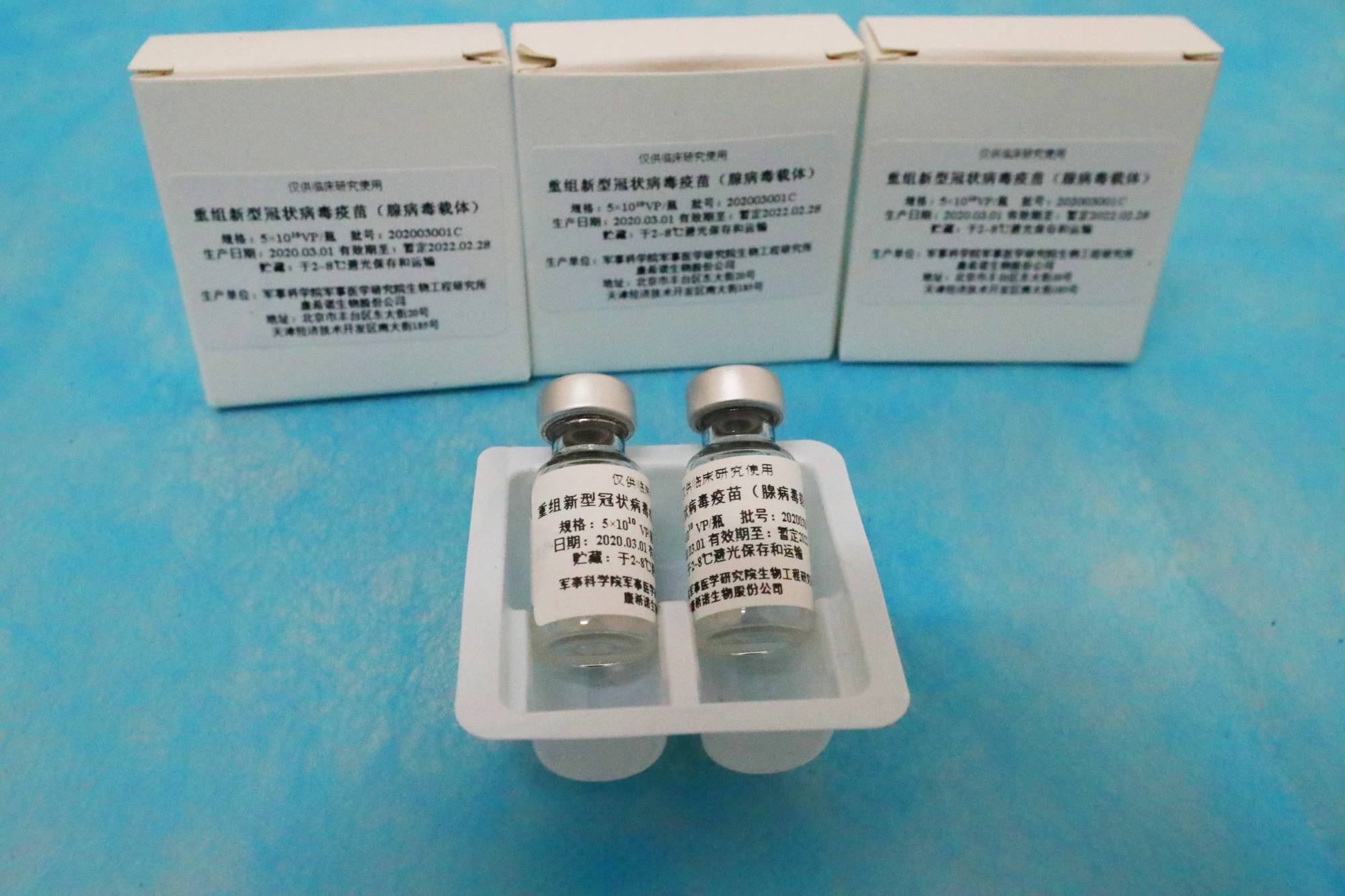
One of the world’s fastest-moving efforts to develop a COVID-19 vaccine is falling behind rivals, its advance appearing to be stymied by political tensions between China and Canada and concerns its shot may not work as well as others.
CanSino Biologics Inc., the Chinese company which in March started the world’s first human tests on an experimental coronavirus shot, has yet to kick off critical final-stage trials on the vaccine it developed with the Chinese military. Meanwhile, rivals like U.S.-based Moderna Inc. and Britain’s AstraZeneca PLC as well as China’s Sinovac Biotech Inc. and Sinopharm are well into this last phase of testing, administering their vaccines on thousands of people to find out if they work.
With its Phase III trials yet to begin, CanSino hasn’t had the opportunity to assuage concerns from earlier-stage data, which showed the immune response generated by its shot varied greatly among participants. Its setbacks offer a look at both the scientific and political incertitudes companies are battling as they race to produce a vaccine against the virus that has already killed more than 850,000 people worldwide.


















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.