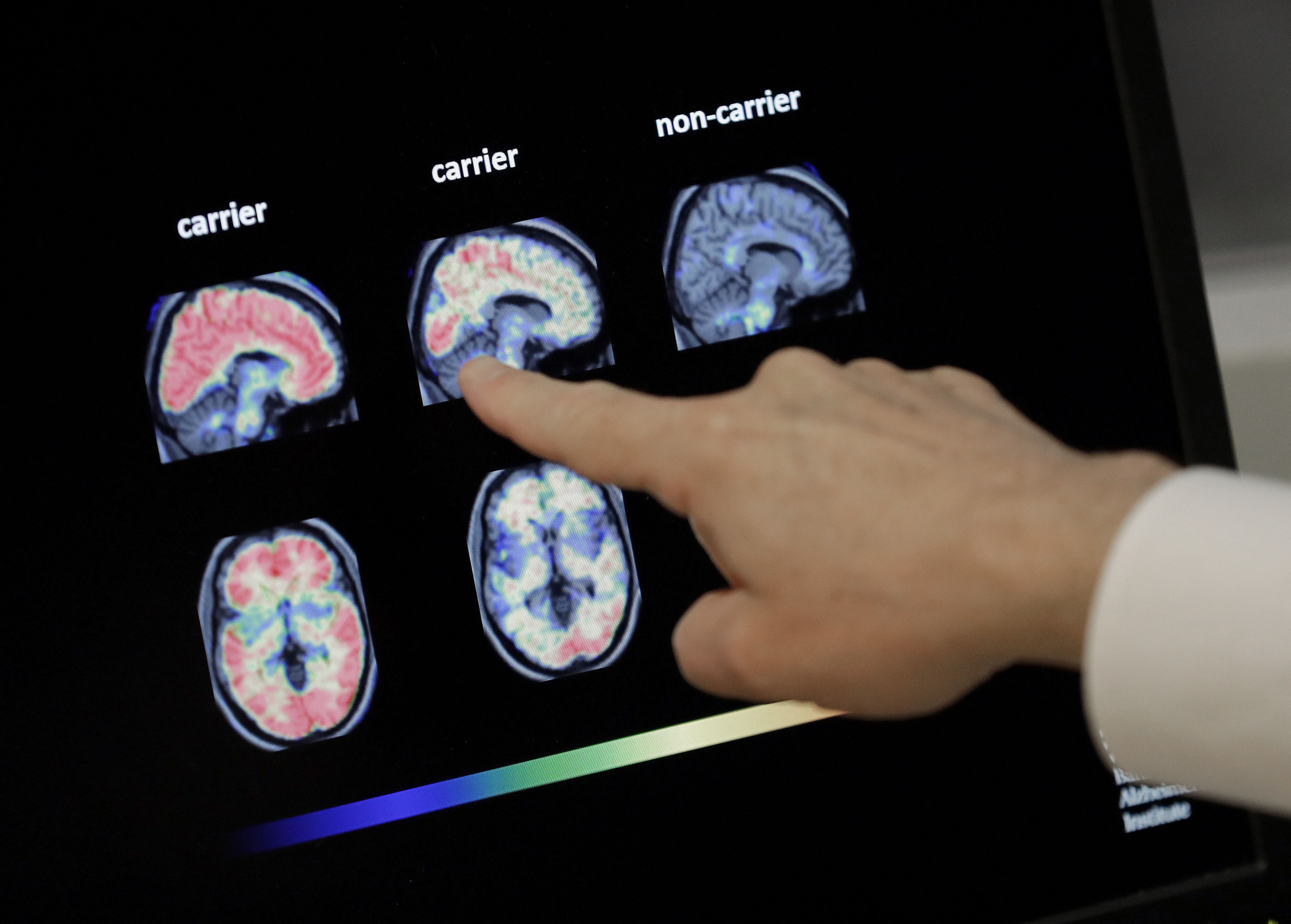
Biogen Inc. revived plans on Tuesday to seek U.S. approval for the Alzheimer's treatment aducanumab and said data from more patients in two discontinued studies showed the drug reduced the decline of patients.
The drugmaker's shares soared 27 percent in New York trading, recouping almost all of the $18 billion it lost when it said in March it was abandoning the two studies. Shares in Eisai Co., Biogen's Japanese partner in the drug, were quoted up 18 percent to the daily limit high of ¥6,534 in Tokyo trading.
The field of experimental Alzheimer's treatments is littered with high-profile failures, with many major drugmakers abandoning the race to develop a medicine for a disease that makes up 60 to 70 percent of an estimated 50 million dementia cases globally.
















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.