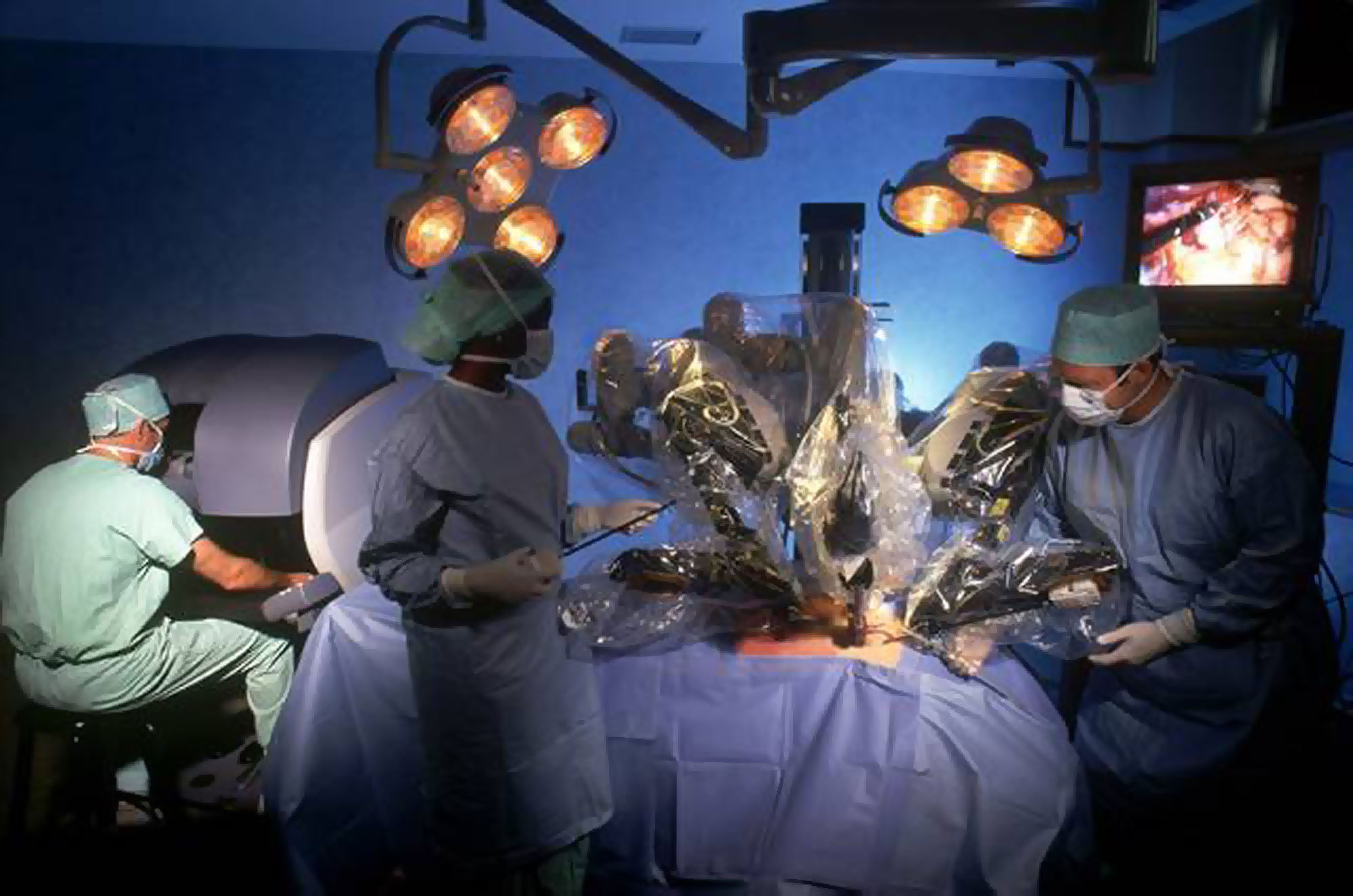
The number of adverse incident reports involving Intuitive Surgical Inc.'s robots has more than doubled this year, according to U.S. regulators, who just released a physician survey showing no consistent training exists for the machines, which are also used in Japan.
The Food and Drug Administration received 3,697 adverse reports through Nov. 3, compared with 1,595 in 2012. The surge, though, doesn't necessarily mean the rate has changed, said William Maisel, with the FDA's device unit. Rising robot use in the United States and Japan, along with recent media reports and recalls, may have spurred public attention, he said. The reports, issued by the company and medical professionals, are largely unverified by the agency.
The FDA survey released Friday included 11 doctors who have performed from 70 to 600 robot surgeries each. While they said the device led to fewer complications and shorter recoveries, they reported incidents in which robot arms collided or missed their mark and noted training was an issue.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.